Why is the value of the secondary anticorrosive layer measured by the coating thickness meter fluctuating
Время выпуска:
When we use the coating thickness meter to measure the anticorrosive layer, the value of the instrument often has little fluctuation, but if it is after the second anticorrosion, there will be a high and low deviation, why is this?
To understand this problem, let's talk about two common corrosive pollutants, rust and oxide. The underlying logic of corrosion is as follows:
Rust is considered a contaminant on the steel surface and must be identified because steel areas that have previously been rusted and then thoroughly cleaned by abrasive sandblasting tend to rerust in the same areas where rust was formed.
The corrosion of iron is electrochemical. In order for iron to corrode, it first needs to ionize, then react with other ions in the region and oxygen in the air, and finally become iron trioxide, which is often referred to as red rust. These corrosion reaction processes are shown in the following formula.
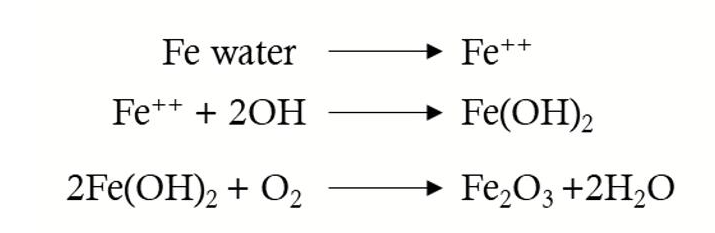
The type of reaction involved in rust formation (i.e. the formation of iron ions and their direct reaction with all moisture to form ferrous hydroxide) is a close reaction on the steel surface.
The anode and cathode regions formed on the steel surface are related to the lattice structure and grain boundaries of the steel. Due to this complex onlooker reaction, the surface of the iron reaction product is difficult to be cleaned up. The surface can be prepared into a white metal state by abrasive sandblasting, but if the prepared surface is placed in a wet environment, it is soon obvious that The area of the previously spoiled ton began to corrode earlier than the other area of the steel surface.

Here we introduce chloride as a typical surface pollutant. Chloride pollution is the most common for almost all surfaces exposed to the Marine environment. It can also be found in the salt industry, the chlor-alkali industry, coal mining and all industries that use soluble chlorides.
The problem with steel and chloride is that ferrous chloride is formed wherever the two materials come into contact. For example, chloride ions are absorbed into the corrosion and adhere to the steel surface. Iron ions, chloride ions and water form a solution of iron chloride, which is not only conductive to help with electrochemical corrosion reactions, but also itself is a strong etchant on the steel surface. When exposed to air, ferrous chloride is oxidized to form ferric chloride. Ferric chloride is a hygroscopic salt that absorbs moisture from the air and forms ferric chloride solution on the rigid surface. In this case, even if the steel has been thoroughly sandblasted, even the small amount of ferric chloride or ferrous chloride remaining on the surface or pitted area will accumulate moisture from the air. This creates a highly concentrated solution of ferric chloride.

Now we observe a steel surface contaminated by chloride and thoroughly sandblasted, observe the rust pit area with a microscope, we can not see the water vapor condensation from the air in the pit area on the steel surface, the condensed liquid almost immediately turned light green, indicating that there is a solution of ferrous oxide, formed water droplets from green to light yellow, surface iron ions have changed, Then, as the solution darkens, iron ions in the solution react with oxygen to form brown deposits, and a later reaction involves the water condensing the bottom and surrounding steel surfaces, turning dark brown or black. Even on surfaces that have been sandblasted two or three times with dry abrasives, this type of reaction still occurs in areas that have eroded, and organic coatings applied to such surfaces tend to fail as iron salts absorb water through the coating through osmosis. And gradually develop adhesion or bubbling areas, this type of surface contamination has caused countless coating failures around the world.
Ключевые слова:
Предыдущая страница
Следующая страница
Предыдущая страница:
Следующая страница:
Мы будем Контакты вас
Успех начинается с сообщения и телефонного звонка